March 06, 2026
By Adjoa Kyerematen
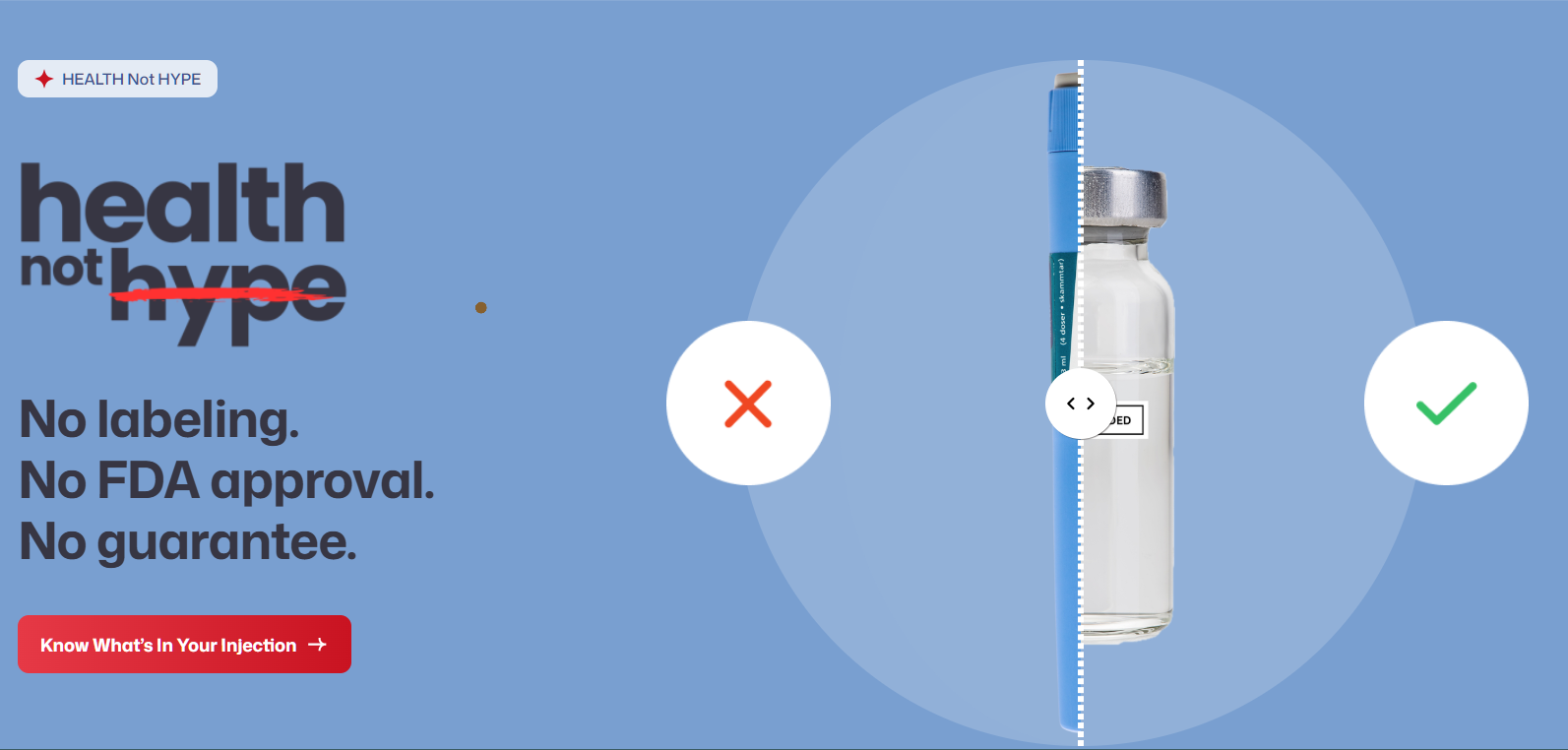
National Minority Quality Forum Launches “Health Not Hype” Campaign During Obesity Care Week to Warn Patients About Risks of Compounded GLP-1 Drugs
Read More

Posted December 6, 2011 | Updated February 6, 2012
By Gary A. Puckrein
Published on the Huffington Post
Today’s advanced medicines, known as biologics, treat some of the most serious and costly medical conditions like cancer, diabetes, and kidney disease. Though it is impossible to make generic versions of these drugs, the 2010 Affordable Care Act has instructed the FDA to figure out how to approve biosimilars — the imitative versions of biologics — for the healthcare marketplace. There is potential for biosimilars reduce healthcare costs, but enacting smart biosimilar regulation will be crucial to ensure patient safety.
Patient safety is duly important for the minority community. At the National Minority Quality Forum, we are focused on improving health care for racial and ethnic communities at higher risk for disease and illness than the general population. We believe in leveraging data and new information to combat disparities in health outcomes with better treatment.
And we believe there are several steps the FDA must take to ensure that these drugs help underserved patients rather than introduce them to new risk.
Enjoy This Post? Read More Articles Below